8162 trials related to COVID-19 were registered in clinicaltrials.gov as of August 24, 2022. I am curious how many of them are related to proteomics. The following content records results of my interest.
I searched on ClinicalTrials.gov using keywords “COVID-19” and “Proteomics”, which returned 39 results. In them, 4 are not yet recruiting, 20 are recruiting, 6 are active yet not recruiting, and 3 are completed. The rest are suspended, terminated, or in an unknown status.
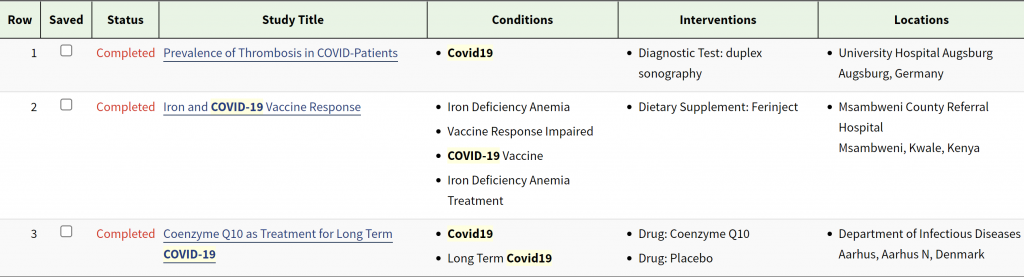
The three completed trials are as follows. None of these trials lasted longer than one year, probably due to the relatively easy patient recruitment in the pandemic and the relatively number of patients recruited are 525, 121, and 121, respectively.
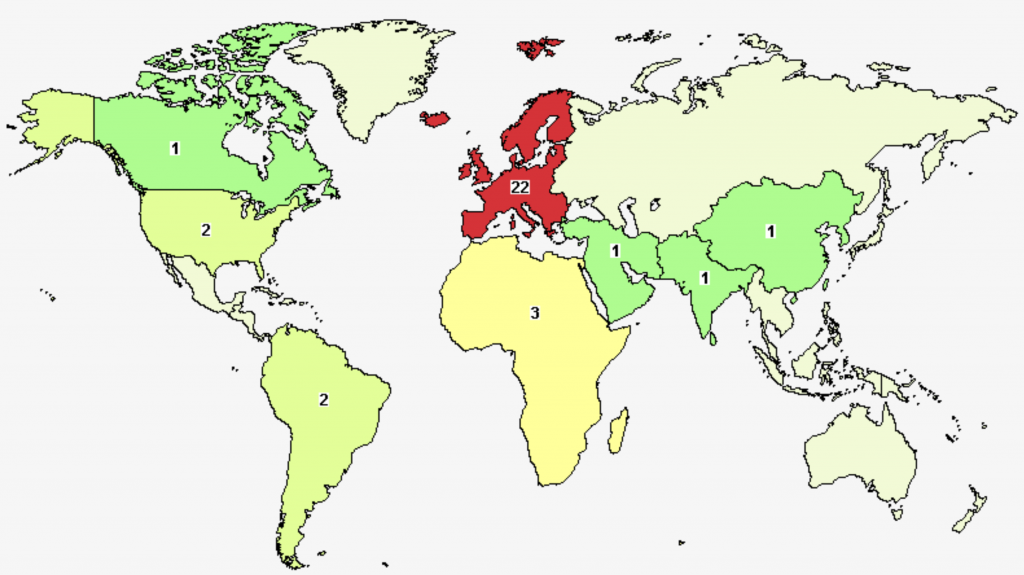
The region/country with the highest number of registered trials was in Europe (n = 22), led by France (n = 7). China, as the only country that had registered a clinical trial in East Asia, had one ongoing (status: recruiting) trail from Wuhan Union Hospital, submitted in June 2021.
This trail from China aims at establishing an artificial intelligence system for intelligent pathological diagnosis and therapeutic effect prediction. Its research object does not only include COVID-19, but also involves tumors and/or other infectious diseases in the respiratory system. Its strategy involves multi-modal data, which includes porteomics of blood, urine, tissues, and exhaled air condensate samples. No result has been posted yet.

In the 39 clinical trials, the main utility for proteomics is to profiling: to investigate the host responses of COVID-19 patients under different status, such as disease severity and drug use. 7 of them mentioned that they may based on their proteomic findings prioritize biomarkers. However, for the time being, none of the trials were evolved directly from previous proteomic findings.